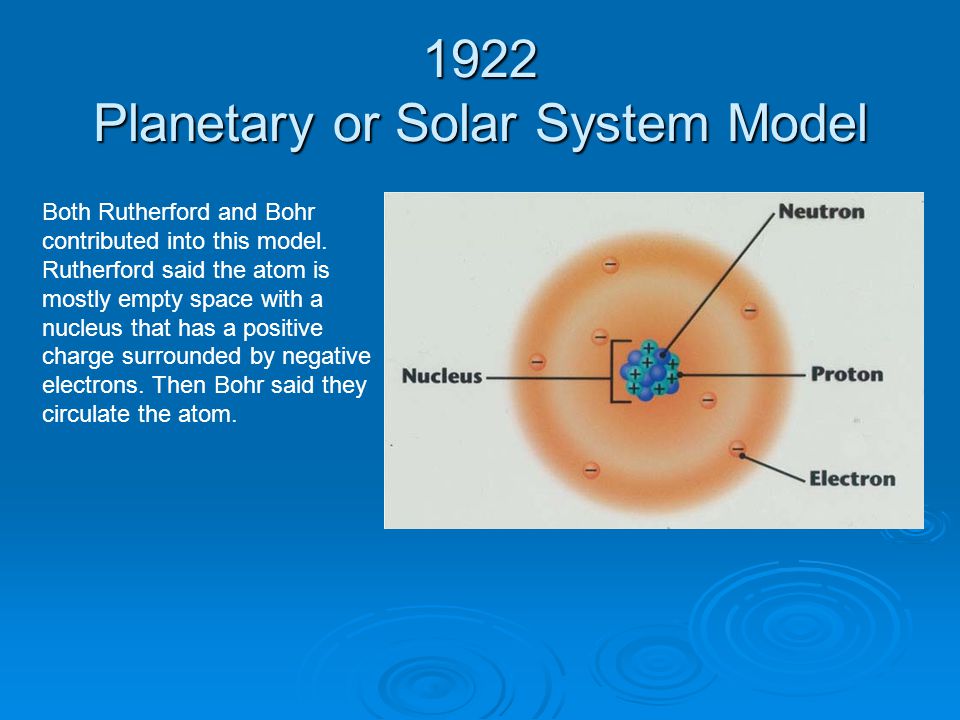
Planetary Or Solar System Model Of The Atom
Planetary or solar system model of the atom. Unfortunately there was a serious flaw in the planetary model. Of any rotating object or collection of objects--even of the Earth Sun or the solar system. Bohrs Model of the Atom.
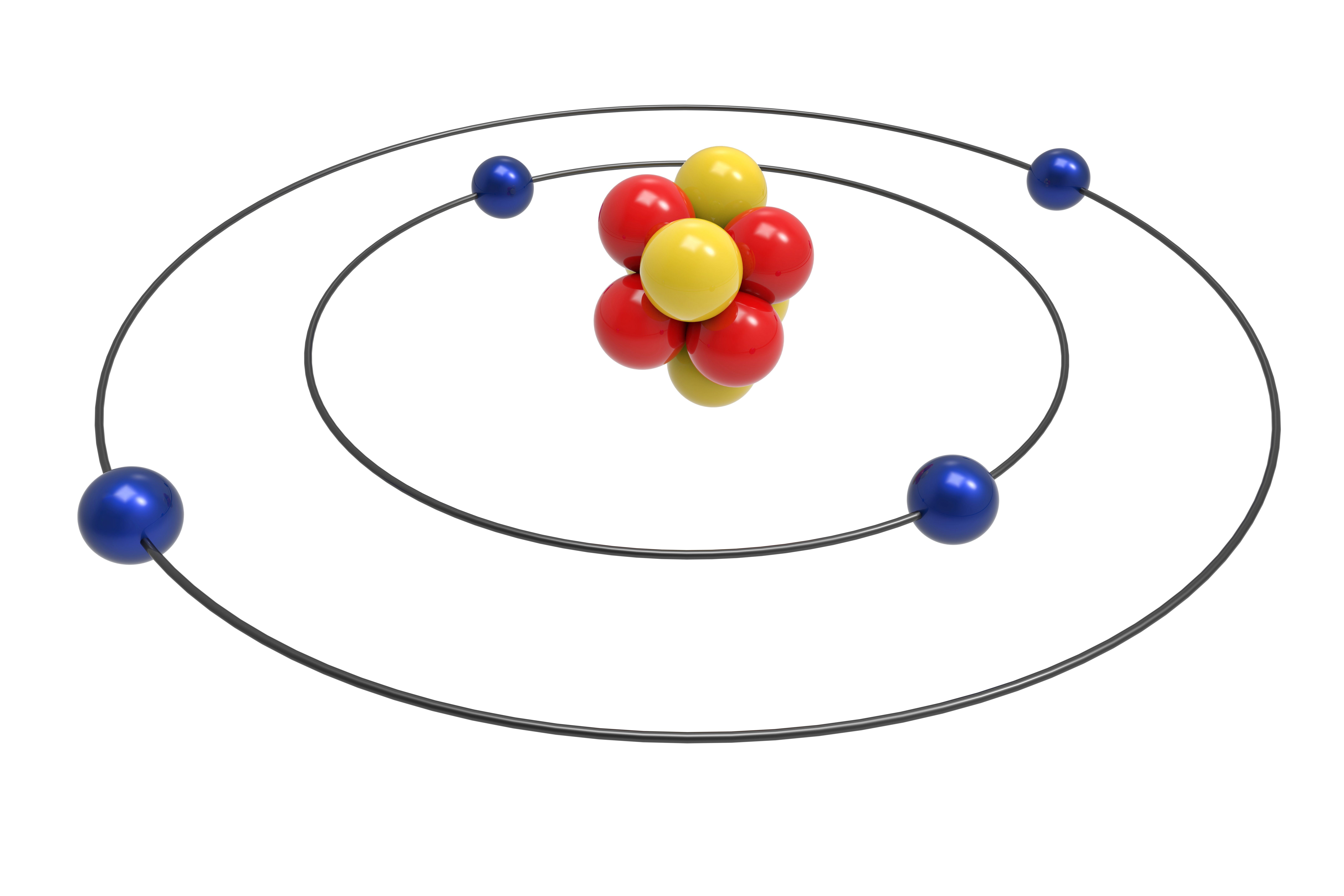
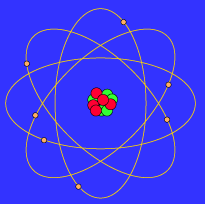
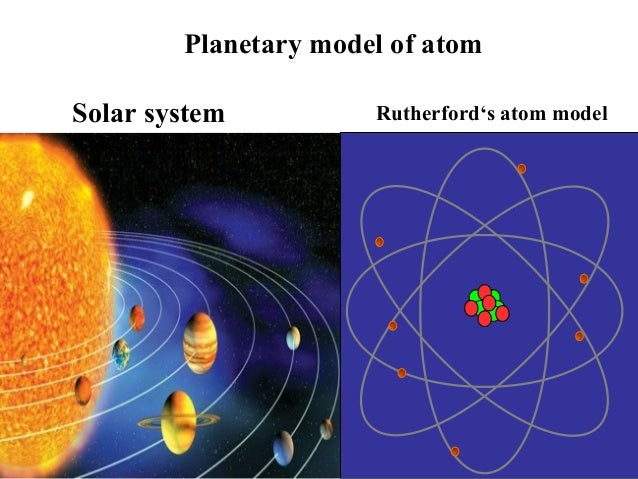
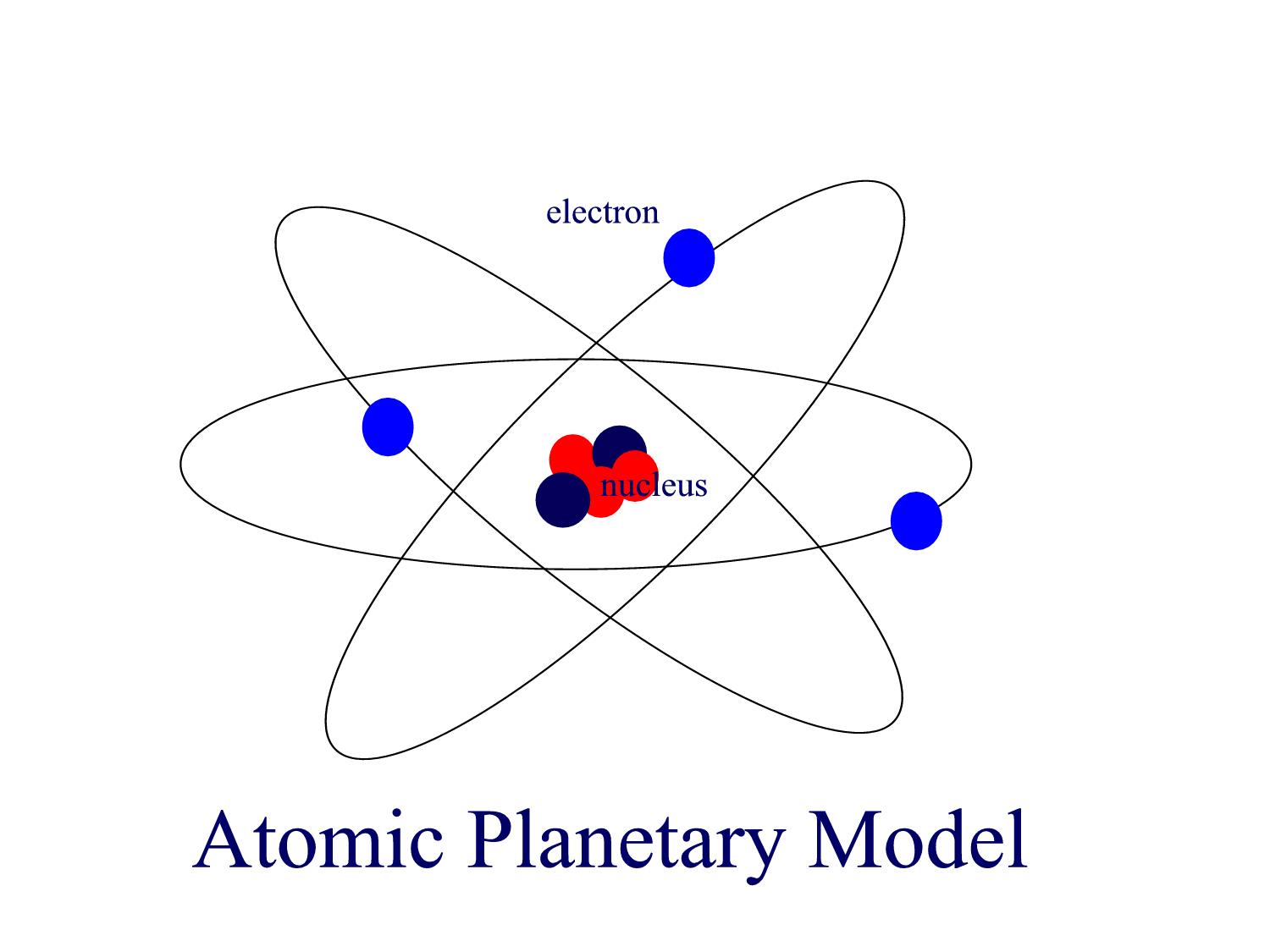
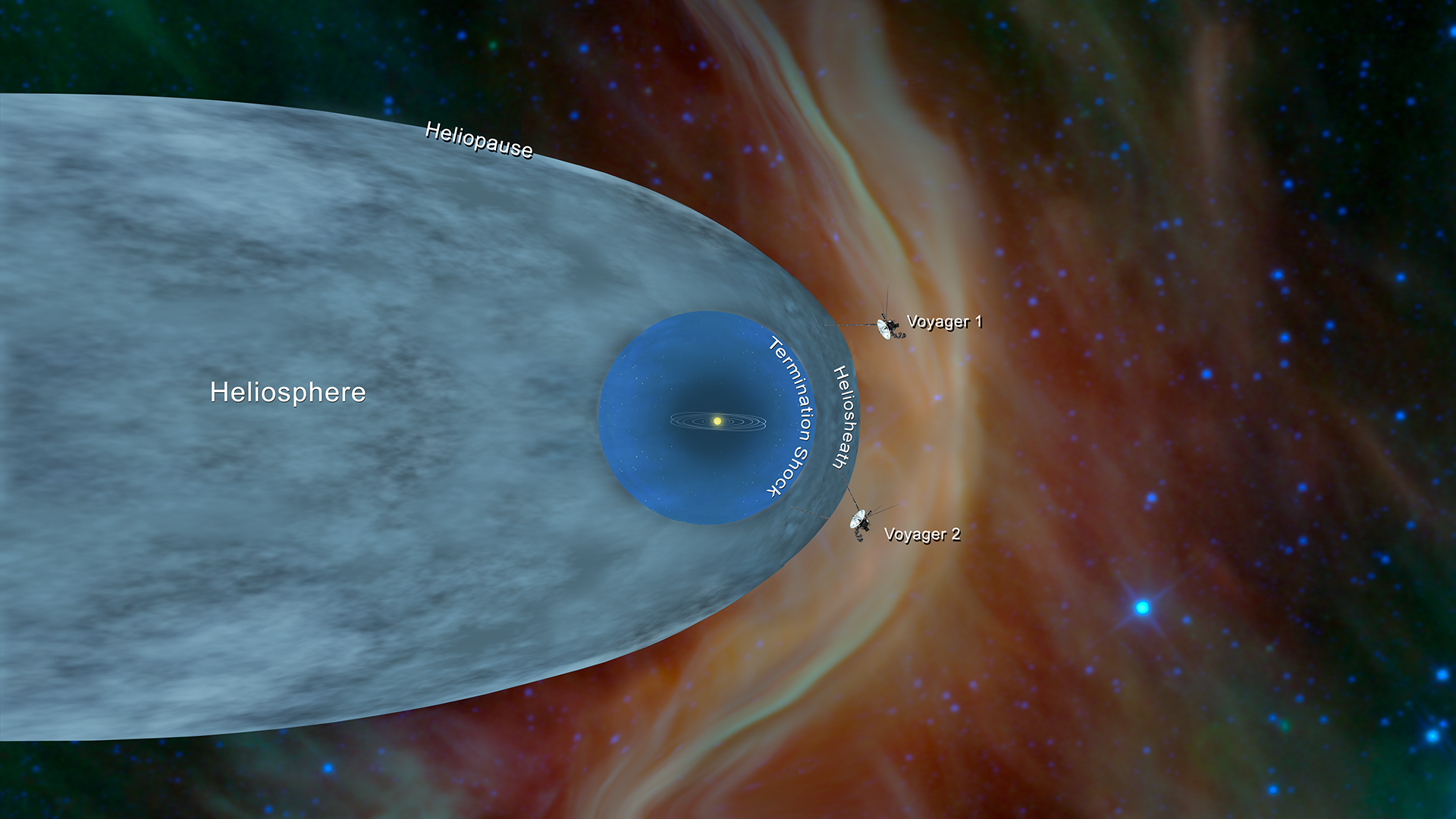
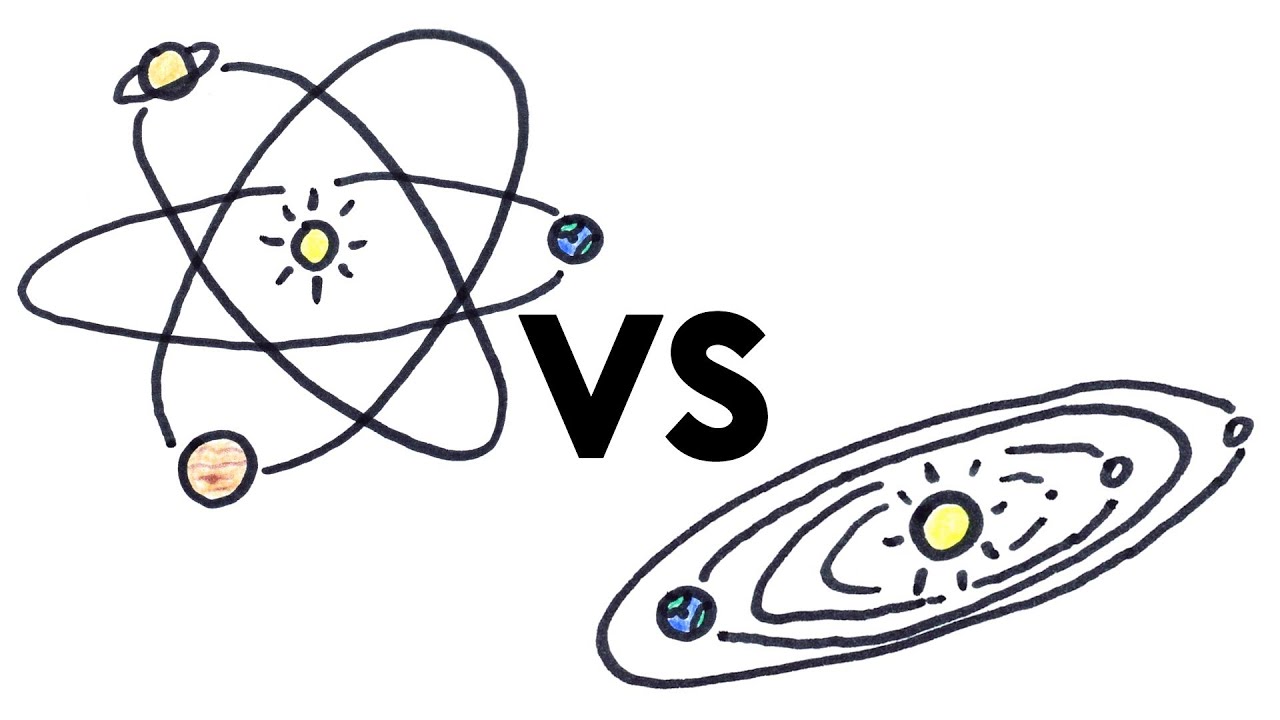
The planetary period of our solar systems planets are well defined and may yet be in transition to a final state in which all other singular stars solar systems will form. But it also kind of suggests that planets might revolve around the sun every which way. Another way of thinking about this model was that the atom was seen to be like a mini solar system where the electrons orbit the nucleus like planets orbiting around the sun.
It measures the total amount of rotation--adjusted for the mass involved its average distance from the. A simplified picture of this is shown alongside. It looked like a miniature planetary system.
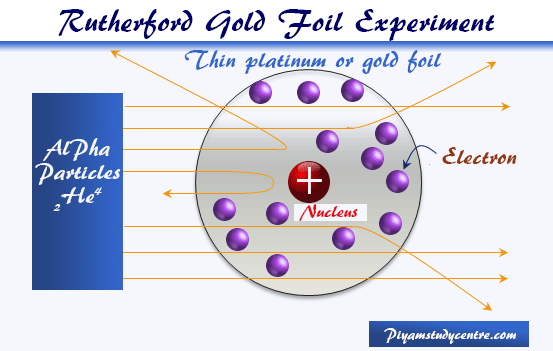
A solar system cant be considered as atom because the structure of atom is not planetaryThe electron position cant be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximumFor more detail study Heisenberg uncertainty principle. Atom Atomic Structure Electrons Ernest Marsden Ernest Rutherford Gold Foil Experiment Hans Geiger JJ Thomson Neutrons Nuclear Atom Nucleus Nucleus of an Atom Planetary Model of Atom Planetary Model of the Atom Plum Pudding Model Protons Rutherford Atomic Model Rutherfords Gold Foil Experiment Solar System Solar System. The solar system model describes an atom as a central massive positive entity the nucleussun and orbiting around it the negative entities the electronsplanets.
There are many theories and studies regarding this Titius-Bode Law The Quantization of Orbits of Planets and Quantum Mechanics Describes Planetary Orbits just to name a few. Niels Bohr with Albert Einstein at Paul Ehrenfests home in Leiden December 1925. Rutherfords atomic model or planetary model of the atom is a model proposed by Ernest Rutherford.
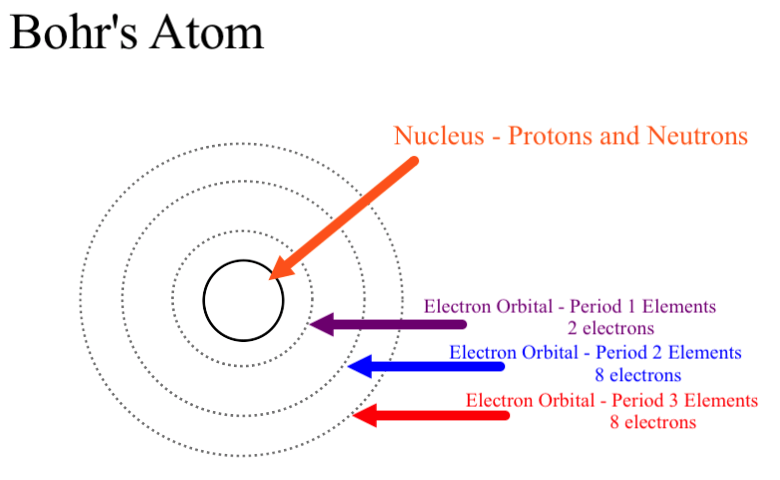
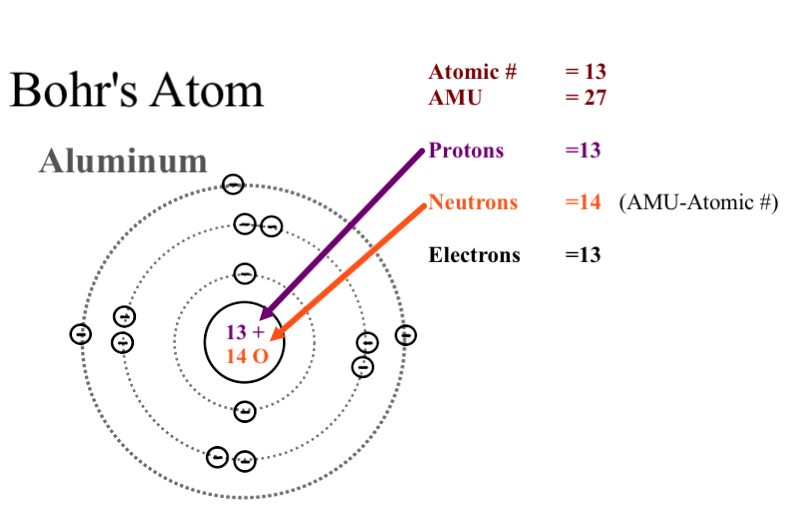
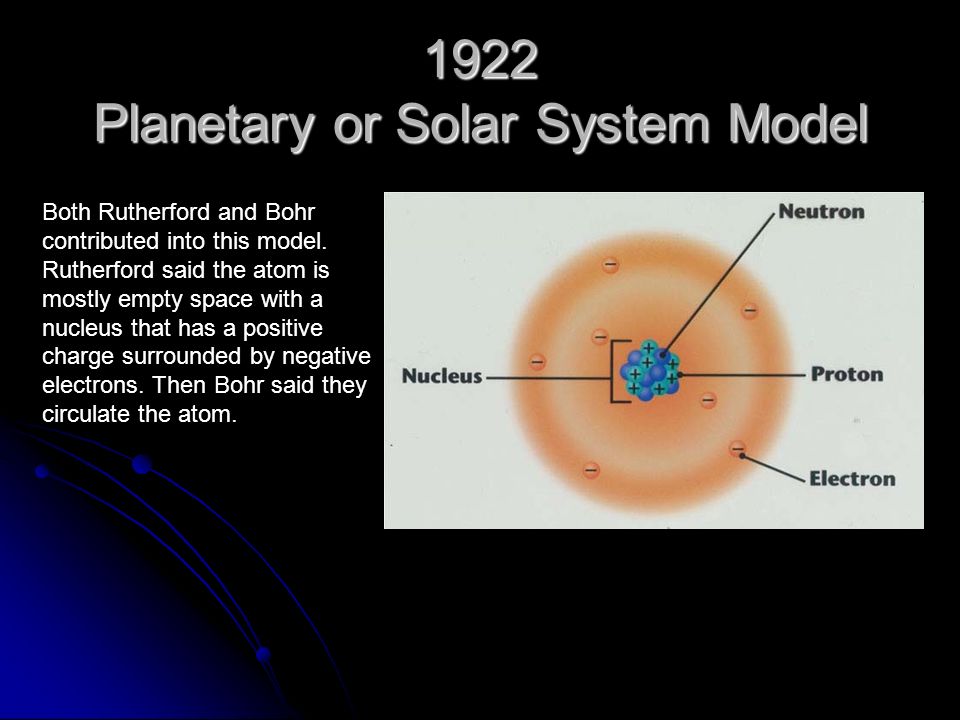
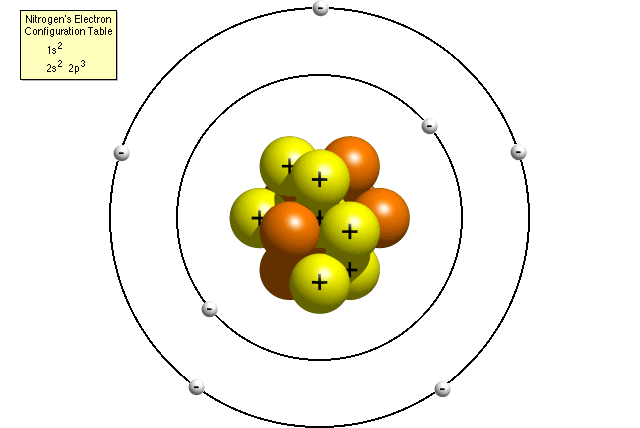
Neils Bohr came up the Solar system model of the atom in 1913. This similarity suggests that electrons should move around the nucleus in well-defined orbits. Then it had the electrons moving around the nucleus in eliptical oval-shaped paths or orbits.
The Bohr model is the Most Accurate Model of an Atom. The atom was first described as an indivisible particle.
Bohrs planetary model of the atom had all of the protons and neutrons clustered together in a nucleus at the center of the atom like most scientists still believe that they are.
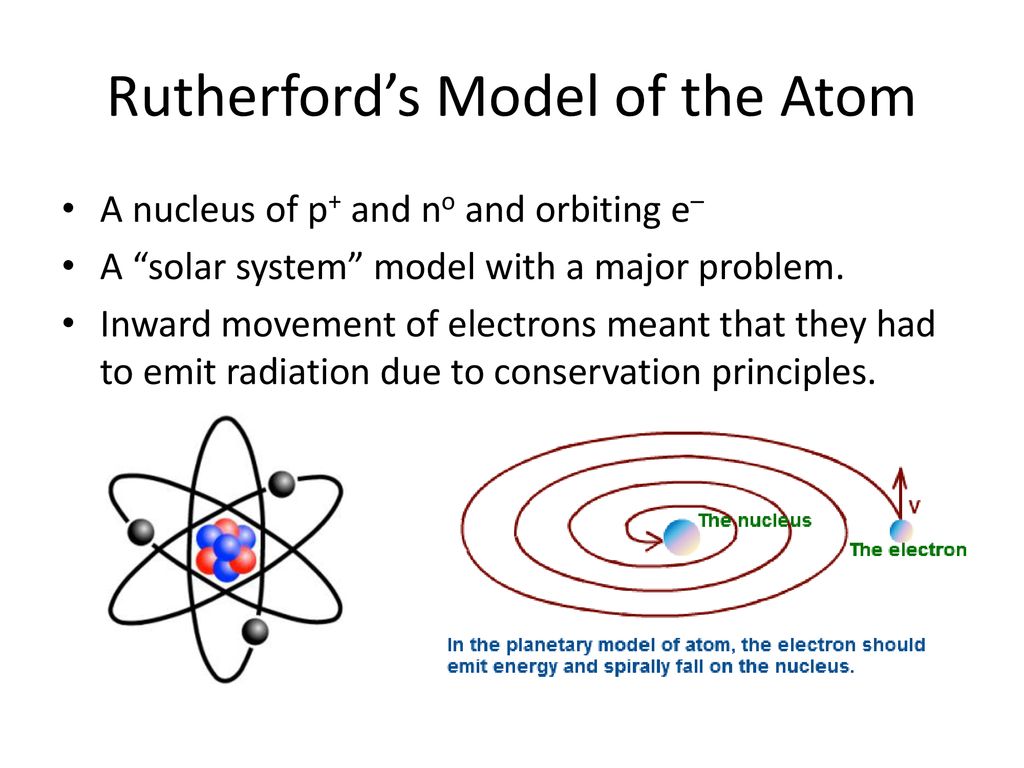
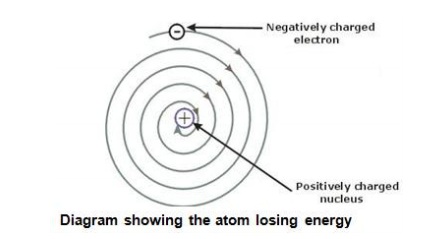
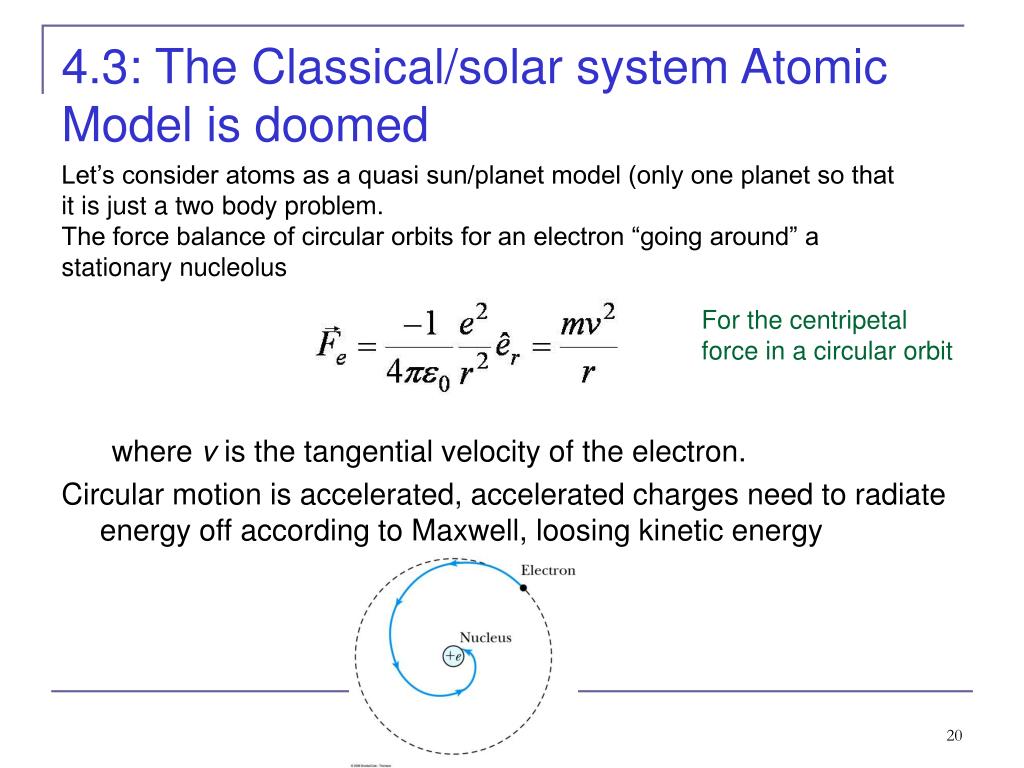
The problem with this is that the electrons are CHARGED particles and moving around in a circle they have CENTRIPETAL acceleration even if they move with constant velocity in. Rutherford model or nuclear planetary model of an atom in chemistry or physics describes by New Zealand born physicist Ernest Rutherford by gold foil experiment on the thin metal atoms. It looked like a miniature planetary system. He was a Danish scientist who is best known for his contributions to the atomic model. Niels Bohr with Albert Einstein at Paul Ehrenfests home in Leiden December 1925. Rutherfords atomic model of an atom is like a small scale solar system. Of any rotating object or collection of objects--even of the Earth Sun or the solar system. Unfortunately there was a serious flaw in the planetary model. The solar system model describes an atom as a central massive positive entity the nucleussun and orbiting around it the negative entities the electronsplanets.
But it also kind of suggests that planets might revolve around the sun every which way. The reason that its called a planetary model is that the. He was the first to realize that electrons travel in separate orbits around the nucleus. The atom was first described as an indivisible particle. So is our solar system somehow special in its flatness or is the planetary model of the atom doubly wrong. Rutherford model also called Rutherford atomic model nuclear atom or planetary model of the atom description of the structure of atoms proposed 1911 by the New Zealand-born physicist Ernest Rutherford. Atom Atomic Structure Electrons Ernest Marsden Ernest Rutherford Gold Foil Experiment Hans Geiger JJ Thomson Neutrons Nuclear Atom Nucleus Nucleus of an Atom Planetary Model of Atom Planetary Model of the Atom Plum Pudding Model Protons Rutherford Atomic Model Rutherfords Gold Foil Experiment Solar System Solar System.



















Post a Comment for "Planetary Or Solar System Model Of The Atom"